
By Richard A. Rawson, Ph.D.
Research Professor, Vermont Center for Behavior and Health
Professor Emeritus, UCLA
The current opioid crisis is arguably the most deadly public health epidemic since the 1918, influenza pandemic in the US. However, as historians and epidemiologists, including the late David Musto from Yale and Jim Hall from Nova University in Miami have noted, specific categories of drug problems in the US tend to cycle over time and there are signs a new cycle is on the horizon. There is clear evidence that we are seeing the rise of, or the continuing serious levels of, psycho-stimulant (cocaine and methamphetamine) use. Jane Maxwell, one of the most respected epidemiologists in the area of drug trends was recently quoted in several recent US News reports on the increasing evidence of escalating use and fatalities from amphetamine/methamphetamine.
The surge in hospitalizations and deaths due to amphetamines “is just totally off the radar,” said Jane Maxwell, an addiction researcher. “Nobody is paying attention.” US News, Nov 26, 2018.
"I've got more meth deaths in Texas than heroin," Maxwell says. US News April 24, 2019.
The opioid epidemic and the more recent upsurge of stimulant use are not independent developments. As noted by NIDA Deputy Director Wilson Compton:
Between individual case reports and broader overdose death data, "we're seeing a larger number of people who are heavily involved in methamphetamine and opioid simultaneously." US News, April 24, 2019.
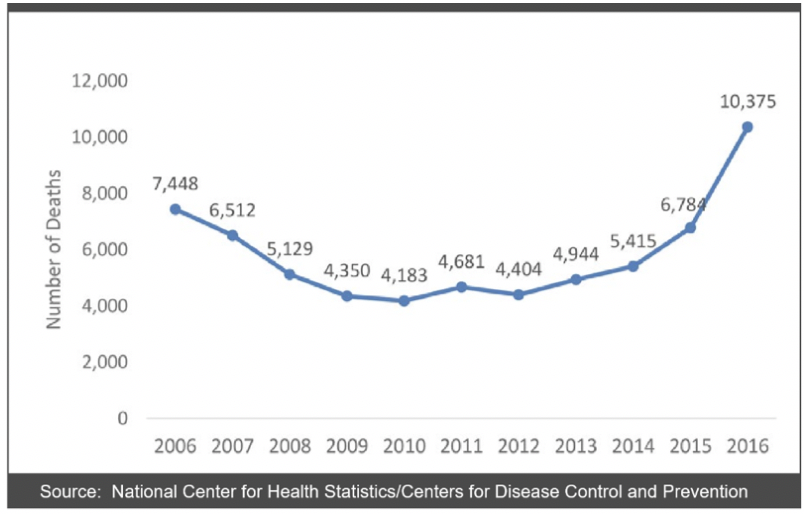
The most clear-cut evidence of the rise of the stimulant-related public health impact in the US is the rapidly escalating rate of deaths associated with stimulants. Deaths due to cocaine increased from 5,908 to 14,257 from 2006-2016 (Amhad et al., 2018[1]) as illustrated in figure 1.
Figure 1. Drug Poisoning Deaths Involving Cocaine, 2006 – 2016.
In parts of the US that have successfully expanded the use of medications for opioid use disorder, there are reports of substantial increases of stimulant use among this patient population. In the Eastern US (and in urban areas generally), cocaine appears to be the most widely used stimulant, while in the West and Midwest (and in more rural areas), methamphetamine is used at an increasing rate among this patient population (Kasper and Cicero, 2018).
Treatment of stimulant intoxication, psychosis and withdrawal
Cocaine and methamphetamine both produce a potent release of dopamine and norepinephrine which leads to euphoria, hyperexcitability, hypersexuality, increased locomotor activity, agitation, loss of appetite and psychotic symptoms including paranoia and hallucinations. The half-life of methamphetamine is 11-12 hours compared with 90 minutes for cocaine (Romanelli & Smith, 2006) and methamphetamine produces more severe and durable physiological and subjective effects than cocaine (Newton et al., 2005). Acute agitation from cocaine/MA intoxication is most often the condition that leads users to seek medical attention, and “talking down” the patient in a calm environment is the first course of action. No medications are available to reverse MA overdose although benzodiazepines may be especially effective in acute management of agitation and distress and may reduce seizure potential.
Psychosis is more common among MA users than with cocaine users and the severity and duration is often greater. The psychotic symptoms frequently include auditory hallucinations, visual (flashing lights, peripheral artifacts), olfactory, and tactile sensations and powerful paranoia and persecutory delusions. Management of stimulant-induced psychosis, which generally is transient, may require use of either a benzodiazepine or an antipsychotic, both of which should be discontinued when acute symptoms have resolved.
Stimulant withdrawal symptoms consist of severe fatigue, cognitive impairment, feelings of depression and anxiety, anergia, confusion, and paranoia. For the majority of patients experiencing acute withdrawal/early-phase abstinence, most symptoms resolve within 2 to 10 days. Rest, exercise, and a healthy diet may be the best management approach for most people in withdrawal. Those with heightened agitation and sleep disturbance may respond to benzodiazepines, but acute depression and anhedonia associated with early abstinence generally resolve without intervention.
Common clinical challenges
There are some common clinical challenges when treating stimulant users. Although not unique to stimulants, cocaine and MA users develop a powerful Pavlovian craving response that is “triggered” when they come into contact with cues previously associated with stimulant use. These cues or “triggers” can include objects (e.g., cash), people (e.g., drug user friends), other substances (e.g., alcohol), places (e.g., areas where stimulants are sold or used), time periods (e.g., weekends, after work) and emotional states (e.g., depression, boredom). This powerful craving response frequently plays a key role in relapse during the initial months of abstinence.
A related aspect of this Pavlovian response concerns the relationship between stimulant use and sexual behavior. Previous research has demonstrated that users of cocaine and MA frequently combine their drug use with sexual activity (Rawson, et al., 2002). During treatment, hypersexuality may continue and can be associated with relapse. In addition, symptoms of depression are very frequent in withdrawal from stimulants and typically abate over the first few weeks of abstinence. However, feelings of anhedonia often persist longer into the first several months of abstinence. Finally, poor engagement and retention of stimulant users in treatment is a frequent challenge. As research has indicated that an extended period of involvement in treatment is associated with better outcomes (Simpson, et al., 1999) this difficulty in retaining patients in treatment is a major challenge.
A number of stimulant user categories and characteristics are noteworthy from a treatment perspective. Women who use stimulants are more likely than men to use stimulants for weight loss and should be screened and treated for eating disorders in order to prevent relapse when normal weight is achieved. Stimulant-using men who have sex with men (MSM) (particularly MA users) are at elevated risk of HIV transmission due to high risk sexual behavior associated with their stimulant use. Individuals that use stimulants heavily (daily) or via injection require more intensive treatment (frequently inpatient/residential level care). These heavier users frequently experience more frequent and severe symptoms of depression, anxiety, and anhedonia and have higher relapse rates than less severe users (Reiber, et al., 2002). Patients in treatment with medication (methadone or buprenorphine) for opioid use disorder (OUD) are also at very high risk for stimulant use disorder.
Medications
After 30 years of intensive effort, there are no FDA-approved medications for treating individuals with stimulant use disorders. There are, however, a number of compounds that have supportive research evidence making them promising candidates, as well as a large number of other possible targets involving all of the systems above for potential treatment of cocaine and methamphetamine users. Promising candidates as medications for cocaine dependence include: Topiramate, modafinil, bupropion, amphetamine, disulfiram, and naltrexone. For MA dependence the following appear to have the most promise: Topiramate, bupropion, mirtazapine, amphetamine/methylphenidate and naltrexone. However, at the present time, none of these medications has definitive evidence of efficacy.
Behavioral Treatments
Behavioral interventions are the mainstay of stimulant use disorder treatment. There are a number of studies in which cocaine and MA users have been treated in the same research trials using the same protocols, personnel and measures. In all of these studies, treatment response of cocaine and MA users has been comparable (Rawson et al; Roll et al.). For this reason, unlike the previous review of medications, we will review the evidence for the following behavioral strategies with the assumption that results from cocaine users will generalize to MA user populations and vice versa.
During the past decade there have been a number of systematic reviews of treatments for stimulant use disorders, including two Cochrane Reviews (Knapp et al. 2009; Minozzi, et al. 2016) a review by the WHO for their Mental health Guidelines document (MH-GAP, WHO) and a meta-analysis by De Crescenzo and colleagues (2018). In all of these analyses, contingency management is recognized as having the strongest evidence of support. For example, in the Knapp et al. (2009) review, the following conclusion is reached from the analysis: “The comparisons between different types of behavioral interventions showed results in favor of treatments with some form of contingency management in respect to both reducing dropouts and lowering cocaine use.” Further, the 2019 meta-analysis (De Crenscenzo, et al. 2018) also concludes that contingency management (together with the community reinforcement approach) produces best evidence of effectiveness for producing a variety of positive outcomes.
Contingency Management (CM) (also known as motivational incentives) applies the principles of positive reinforcement for performance of desired behaviors consistent with abstinence from cocaine or MA. CM involves the contingent delivery of an incentive for behaviors such as attendance at treatment sessions or a drug-negative urine specimen or documented completion of a homework assignment. Incentives include desired items or privileges, such as vouchers. There are a variety of ways to structure and individualize CM and a variable schedule of reinforcement can be applied, using the “fishbowl approach” (Petry et al., 2000). This relatively simple positive reinforcement procedure has been shown to produce and sustain substantial and clinically meaningful reductions in stimulant use.
Some of the specific research findings supporting contingency management for the treatment of stimulant use disorders include the landmark paper by Higgins et al. (1991) that documented highly significant reductions in cocaine use and very large and significant increases in extended periods of cocaine abstinence using CM. Roll and colleagues (2005) extended these findings to MA users and reported that CM produced significantly greater retention in treatment and significantly more MA-negative urine samples. Rawson et al. (2002) found that with individuals in methadone treatment who also used cocaine, CM produced significantly more cocaine-free urine samples than no treatment (other than methadone) or cognitive behavioral therapy (CBT). Further, the addition of CBT did not produce additional benefits over and above CM alone.
Although there is strong empirical support for CM, its application in real world treatment settings has been limited. One effort that has shown promise in extending its application is a large trial promoting the use of CM as a routine treatment approach in the VA. The effectiveness of this implementation project has been documented DePhilippis and colleagues (2018), who reported that CM could be successfully implemented across a large number of sites and that patient outcomes were significantly improved by the addition of CM within these treatment settings.
Community Reinforcement Approach (CRA) is a combination of behavioral strategies that address the role of environmental contingencies in encouraging or discouraging drug use, and attempts to rearrange these contingencies so that a non-drug using lifestyle is more rewarding than a using one. CRA components include: behavioral skills training, social and recreational counseling; marital therapy; motivational enhancement; job counseling and relapse prevention. In a number of the CRA trials for cocaine use disorder, a voucher-based CM reinforcement program was added.
To isolate the effects of CRA, Higgins et al. (2003) compared CRA with vouchers vs vouchers only over a 24 week study period. Study results demonstrated that while both conditions produced significant reductions in cocaine use, participants in the CRA plus vouchers condition were retained better in treatment and had fewer days of cocaine or alcohol use. Further, those treated with CRA plus vouchers had more employed days, fewer hospital admissions or legal problems and reduced depressive symptoms. Therefore, while vouchers (CM) have been well established as an efficacious treatment for stimulant use disorder, the addition of CRA can provide additional benefits, as a systematic review concluded (Roozen et al., 2004).
Cognitive behavior therapy (CBT) is a form of “talk therapy” based on principles of social learning theory that is used to teach, encourage, and support individuals in reducing or stopping their harmful drug use (Carroll, 1998). CBT provides skills that are valuable in assisting people in gaining initial abstinence from drugs (or in reducing their drug use) and helping people sustain abstinence. CBT addresses negative thought patterns and teaches individuals how to cope with distress in order to prevent relapse. A systematic review highlighting randomized control trials using CBT as an intervention for MA users reported that CBT was associated with reduced stimulant use and facilitated improvements in mood and other areas of functioning (Lee and Rawson, 2008) and a review of CBT for a variety of substance use disorders concluded that CBT is an effective treatment approach (Dutra, 2008). Carroll and colleagues conducted studies establishing the efficacy of cognitive behavioral therapy (CBT) for the treatment of cocaine use disorder (Carroll et al., 1994 a,b). These studies demonstrated that the use of their CBT manual reduced cocaine use over a 1-year period. In fact, their report suggests that CBT produces especially efficacious results at follow-up points. In a meta-analysis of behavioral treatments for cocaine and methamphetamine use disorders (De Crescendo et al., 2018), studies evaluating efficacy of CBT consistently reflect positive findings. Data from this meta-analysis also indicated, however, that CM strategies consistently result in greater reductions in stimulant use when compared to CBT.
Recently, CBT has become more accessible through computerized delivery (CBT for CBT) (Carroll et al., 2008). In a randomized trial for cocaine using individuals in methadone maintenance treatment, results showed that participants receiving computerized CBT for CBT were significantly more likely to have three or more consecutive weeks of abstinence from cocaine compared to controls (Carroll et al., 2014).
The following behavioral strategies have been the subject of at least one randomized clinical trial demonstrating superior outcomes when compared to control procedures: Exercise therapy, transcranial magnetic stimulation (TMS), mindfulness, twelve-step facilitation, and matrix model.
There is clearly a re-emergence of cocaine and methamphetamine use in the US concurrent with the existing opioid epidemic. There are established medical treatments for stimulant intoxication, psychosis and withdrawal. There are some specific clinical issues and populations that require special attention when developing clinical strategies for the treatment of individuals with stimulant use disorders. While the FDA has not approved any pharmacotherapy to treat stimulant use disorders, behavioral interventions including contingency management (CM), community reinforcement approach (CRA), cognitive behavioral therapy (CBT) have established efficacy in treating individuals with stimulant use disorders.
Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2018. Accessed November 2018 at https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
(Source: U.S. Department of Justice Drug Enforcement Administration. [2018, October]. 2018 National Drug Threat Assessment. [Page 46] https://www.dea.gov/documents/2018/10/02/2018-national-drug-threat-assessment-ndta)